Adhésion cellulaire et mécanique
BENOIT LADOUX & RENÉ MARC MÈGE
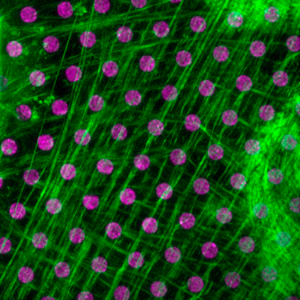
Les contraintes mécaniques et la transmission des forces jouent un rôle essentiel dans les organismes vivants multicellulaires. Elles régulent des processus biologiques fondamentaux tels que la morphogenèse, les métastases tumorales et la réparation des tissus. Les adhésions cellulaires, couplées au cytosquelette contractile, sont des sites majeurs de transmission de force dans les cellules. Ce couplage mécanique, qui permet aux cellules de détecter et répondre aux changements physiques de l’environnement, a cependant été largement sous-étudié. Dans ce contexte, nous étudions la coopération entre l’adhésion, la signalisation mécanique et biochimique dans l’adaptation des cellules aux changements de leur environnement physique à différentes échelles, de la molécule unique aux tissus.
Mots-clés : Mécanobiologie ; Mécanosensitivité ; homéostasie de l’épithélium ; migration collectives ; mifrofabrication ; biophysique ; extrusion cellulaire ; mécanique des tissues
+33 (0)1 57 27 80 71 / +33 (0)1 57 27 80 67 Contact Benoît LADOUX / René-Marc MEGE @bladoux.bsky.social / @rmmege.bsky.social https://ladoux-mege-lab.cnrs.fr/
A l’échelle de la cellule unique
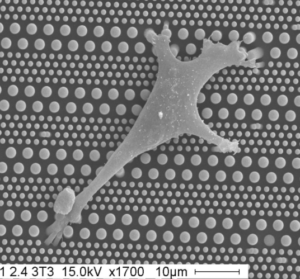 Nous analysons la mécanosensation et la mécanotransduction des adhésions cellule-matrice extracellulaire associées au intégrines, et cellule-cellule associées au cadhérines. Pour répondre à ces questions, nous avons développé des modèles de cellules uniques et de doublets de cellules permettant un contrôle de la formation des adhésions dans un microenvironnement physique et mécanique défini. Couplées à des approches de microscopie avancée, au développement de capteurs de force et à la biologie cellulaire classique, ces approches nous permettent d’étudier les mécanismes moléculaires qui contrôlent le remodelage des adhésions et du cytosquelette, de la forme et de la migration des cellules et leur adaptation à la rigidité de l’environnement ainsi qu’aux propriétés viscoélastiques du cytosquelette et à la tension interne générée par les moteurs à myosine.
Nous analysons la mécanosensation et la mécanotransduction des adhésions cellule-matrice extracellulaire associées au intégrines, et cellule-cellule associées au cadhérines. Pour répondre à ces questions, nous avons développé des modèles de cellules uniques et de doublets de cellules permettant un contrôle de la formation des adhésions dans un microenvironnement physique et mécanique défini. Couplées à des approches de microscopie avancée, au développement de capteurs de force et à la biologie cellulaire classique, ces approches nous permettent d’étudier les mécanismes moléculaires qui contrôlent le remodelage des adhésions et du cytosquelette, de la forme et de la migration des cellules et leur adaptation à la rigidité de l’environnement ainsi qu’aux propriétés viscoélastiques du cytosquelette et à la tension interne générée par les moteurs à myosine.
A l’échelle multicellulaire
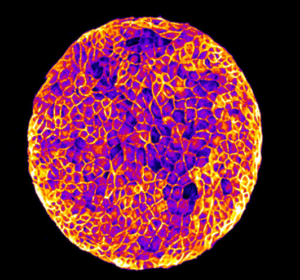 Nous étudions le comportement collectif des cellules dans les feuillets épithéliaux, dans le contexte de l’homéostasie tissulaire et de la cicatrisation. Pour répondre à ces questions, nous développons des outils microfabriqués et des outils biophysiques pour mesurer et contrôler les propriétés mécaniques et la topologie du microenvironnement des cellules. Ces outils sont combinés à des approches moléculaires, des techniques avancées de microscopie optique, d’analyse d’images et de modélisation pour étudier l’influence des propriétés physiques de l’environnement sur l’organisation des couches épithéliales, la migration collective des cellules, la polarisation des cellules individuelles et collectives, la division cellulaire et l’extrusion des cellules. Nous caractérisons comment les contraintes physiques peuvent conduire à des propriétés dynamiques et mécaniques émergentes de divers tissus épithéliaux.
Nous étudions le comportement collectif des cellules dans les feuillets épithéliaux, dans le contexte de l’homéostasie tissulaire et de la cicatrisation. Pour répondre à ces questions, nous développons des outils microfabriqués et des outils biophysiques pour mesurer et contrôler les propriétés mécaniques et la topologie du microenvironnement des cellules. Ces outils sont combinés à des approches moléculaires, des techniques avancées de microscopie optique, d’analyse d’images et de modélisation pour étudier l’influence des propriétés physiques de l’environnement sur l’organisation des couches épithéliales, la migration collective des cellules, la polarisation des cellules individuelles et collectives, la division cellulaire et l’extrusion des cellules. Nous caractérisons comment les contraintes physiques peuvent conduire à des propriétés dynamiques et mécaniques émergentes de divers tissus épithéliaux.
Au niveau des tissus et des organoïdes
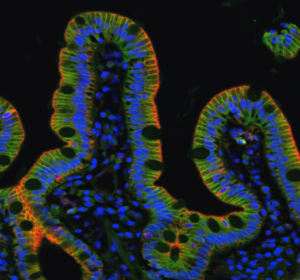 Nous étudions comment des tissus épithéliaux plus complexes, formés de populations de cellules différentes (cellules normales/déficientes pour l’adhésion, normales/cancéreuses, différenciées/cellules souches) confrontées à des substrats homogènes ou hétérogènes (chimie de la matrice extracellulaire, rigidité, géométrie et topographie), régulent l’homéostasie, se séparent et/ou s’auto-assemblent. Nos objectifs sont de déterminer i) comment les contraintes physiques du microenvironnement modulent les propriétés mécaniques des cellules et des tissus épithéliaux, ii) comment elles dirigent une variété de comportements cellulaires incluant la prolifération des cellules souches, l’extrusion ou la délamination de cellules, la migration cellulaire, la différenciation et la polarité, et iii) comment elles ont un impact sur la morphogenèse des tissus épithéliaux normaux, ainsi que sur le développement pathologique des maladies rares de l’intestin. Les substrats biomimétiques couplés à l’imagerie à haute résolution et à la biochimie sont essentiels pour atteindre ces objectifs.
Nous étudions comment des tissus épithéliaux plus complexes, formés de populations de cellules différentes (cellules normales/déficientes pour l’adhésion, normales/cancéreuses, différenciées/cellules souches) confrontées à des substrats homogènes ou hétérogènes (chimie de la matrice extracellulaire, rigidité, géométrie et topographie), régulent l’homéostasie, se séparent et/ou s’auto-assemblent. Nos objectifs sont de déterminer i) comment les contraintes physiques du microenvironnement modulent les propriétés mécaniques des cellules et des tissus épithéliaux, ii) comment elles dirigent une variété de comportements cellulaires incluant la prolifération des cellules souches, l’extrusion ou la délamination de cellules, la migration cellulaire, la différenciation et la polarité, et iii) comment elles ont un impact sur la morphogenèse des tissus épithéliaux normaux, ainsi que sur le développement pathologique des maladies rares de l’intestin. Les substrats biomimétiques couplés à l’imagerie à haute résolution et à la biochimie sont essentiels pour atteindre ces objectifs.
Team leaders
 Benoit LADOUX, Chercheur, LADOUX/MEGE LAB+33 (0)1 57 27 80 71, room 142B
Benoit LADOUX, Chercheur, LADOUX/MEGE LAB+33 (0)1 57 27 80 71, room 142B Rene-Marc MEGE, Chercheur, LADOUX/MEGE LAB+33 (0)1 57 27 80 67, room 142B
Rene-Marc MEGE, Chercheur, LADOUX/MEGE LAB+33 (0)1 57 27 80 67, room 142B
Members
 Lucas ANGER, Doctorant, LADOUX/MEGE LAB+33 (0)1 57 27 80 67, room 142B
Lucas ANGER, Doctorant, LADOUX/MEGE LAB+33 (0)1 57 27 80 67, room 142B Gregory ARKOWITZ, Doctorant, LADOUX/MEGE LAB+33 (0)1 57 27 80 71, room 142B
Gregory ARKOWITZ, Doctorant, LADOUX/MEGE LAB+33 (0)1 57 27 80 71, room 142B Manon ARNAUD, Ingénieure en biologie, LADOUX/MEGE LAB+33 (0)1 57 27 80 67, room 142B
Manon ARNAUD, Ingénieure en biologie, LADOUX/MEGE LAB+33 (0)1 57 27 80 67, room 142B Alexandre BINA, Stagiaire, LADOUX/MEGE LAB
Alexandre BINA, Stagiaire, LADOUX/MEGE LAB Ranjith Kumar CHILUPURI, Doctorant, LADOUX/MEGE LAB+33 (0)1 57 27 80 67, room 142B
Ranjith Kumar CHILUPURI, Doctorant, LADOUX/MEGE LAB+33 (0)1 57 27 80 67, room 142B Joseph D ALESSANDRO, Chercheur, LADOUX/MEGE LAB+33 (0)1 57 27 80 48, room 142B
Joseph D ALESSANDRO, Chercheur, LADOUX/MEGE LAB+33 (0)1 57 27 80 48, room 142B Tien DANG, Ingénieure en biologie, LADOUX/MEGE LAB+33 (0)1 57 27 80 68, room 142B
Tien DANG, Ingénieure en biologie, LADOUX/MEGE LAB+33 (0)1 57 27 80 68, room 142B Cecile Daubech, Doctorante, LADOUX/MEGE LAB
Cecile Daubech, Doctorante, LADOUX/MEGE LAB Simon DE BECO, Enseignant-chercheur, LADOUX/MEGE LAB+33 (0)1 57 27 80 71, room 142B
Simon DE BECO, Enseignant-chercheur, LADOUX/MEGE LAB+33 (0)1 57 27 80 71, room 142B Olivier DESTRIAN, Chercheur, LADOUX/MEGE LAB+33 (0)1 57 27 80 67, room 142B
Olivier DESTRIAN, Chercheur, LADOUX/MEGE LAB+33 (0)1 57 27 80 67, room 142B Sushil DUBEY, Postdoctorant, LADOUX/MEGE LAB+33 (0)1 57 27 80 67, room 142B
Sushil DUBEY, Postdoctorant, LADOUX/MEGE LAB+33 (0)1 57 27 80 67, room 142B Marc Antoine FARDIN, Chercheur, LADOUX/MEGE LAB+33 (0)1 57 27 80 67, room 142B
Marc Antoine FARDIN, Chercheur, LADOUX/MEGE LAB+33 (0)1 57 27 80 67, room 142B Victoria Guglielmotti, Doctorante, LADOUX/MEGE LAB
Victoria Guglielmotti, Doctorante, LADOUX/MEGE LAB Pan JIANG, Postdoctorant, LADOUX/MEGE LAB+33 (0)1 57 27 80 71, room 142B
Pan JIANG, Postdoctorant, LADOUX/MEGE LAB+33 (0)1 57 27 80 71, room 142B Sixtine JOUFFROY, Stagiaire, LADOUX/MEGE LAB
Sixtine JOUFFROY, Stagiaire, LADOUX/MEGE LAB Satish KAILASAM MANI, Postdoctorant, LADOUX/MEGE LAB+33 (0)1 57 27 80 67, room 142B
Satish KAILASAM MANI, Postdoctorant, LADOUX/MEGE LAB+33 (0)1 57 27 80 67, room 142B Maeva LECOQ, Stagiaire, LADOUX/MEGE LAB
Maeva LECOQ, Stagiaire, LADOUX/MEGE LAB Carine ROSSE, Chercheur, LADOUX/MEGE LAB+33 (0)1 57 27 80 67, room 142B
Carine ROSSE, Chercheur, LADOUX/MEGE LAB+33 (0)1 57 27 80 67, room 142B Andreas SCHONIT, Doctorant, LADOUX/MEGE LAB+33 (0)1 57 27 80 71, room 142B
Andreas SCHONIT, Doctorant, LADOUX/MEGE LAB+33 (0)1 57 27 80 71, room 142B Yuan SHEN, Postdoctorant, LADOUX/MEGE LAB+33 (0)1 57 27 80 68, room 142B
Yuan SHEN, Postdoctorant, LADOUX/MEGE LAB+33 (0)1 57 27 80 68, room 142B Clémence THIANT, Doctorante, LADOUX/MEGE LAB+33 (0)1 57 27 80 95, room 142B
Clémence THIANT, Doctorante, LADOUX/MEGE LAB+33 (0)1 57 27 80 95, room 142B Hélène VIGNES, Postdoctorante, LADOUX/MEGE LAB+33 (0)1 57 27 80 67, room 142B
Hélène VIGNES, Postdoctorante, LADOUX/MEGE LAB+33 (0)1 57 27 80 67, room 142B Fanny WODRASCKA, Doctorante, LADOUX/MEGE LAB+33 (0)1 57 27 80 71, room 142B
Fanny WODRASCKA, Doctorante, LADOUX/MEGE LAB+33 (0)1 57 27 80 71, room 142B Wang XI, Chercheur, LADOUX/MEGE LAB+33 (0)1 57 27 80 71, room 142B
Wang XI, Chercheur, LADOUX/MEGE LAB+33 (0)1 57 27 80 71, room 142B
Pour contacter un membre de l’équipe par mail : prenom.nom@ijm.fr
Balasubramaniam L, Doostmohammadi A, Saw TB, Narayana GHNS, Mueller R, Dang T, Thomas M, Gupta S, Sonam S, Yap AS, Toyama Y, Mège RM, Yeomans JM, Ladoux B. Investigating the nature of active forces in tissues reveals how contractile cells can form extensile monolayers. Nat Mater. 2021 Aug;20(8):1156-1166. doi: 10.1038/s41563-021-00919-2. Epub 2021 Feb 18. Erratum in: Nat Mater. 2021 Mar 9;: PMID: 33603188; PMCID: PMC7611436.
Gaston C, De Beco S, Doss B, Pan M, Gauquelin E, D’Alessandro J, Lim CT, Ladoux B, Delacour D. EpCAM promotes endosomal modulation of the cortical RhoA zone for epithelial organization. Nat Commun. 2021 Apr 13;12(1):2226. doi: 10.1038/s41467-021-22482-9. PMID: 33850145; PMCID: PMC8044225.
Jain S, Cachoux VML, Narayana GHNS, de Beco S, D’Alessandro J, Cellerin V, Chen T, Heuzé ML, Marcq P, Mège RM, Kabla AJ, Lim CT, Ladoux B. The role of single cell mechanical behavior and polarity in driving collective cell migration. Nat Phys. 2020 Jul;16(7):802-809. doi: 10.1038/s41567-020-0875-z. Epub 2020 May 4. PMID: 32641972; PMCID: PMC7343533.
Le AP, Rupprecht JF, Mège RM, Toyama Y, Lim CT, Ladoux B. Adhesion-mediated heterogeneous actin organization governs apoptotic cell extrusion. Nat Commun. 2021 Jan 15;12(1):397. doi: 10.1038/s41467-020-20563-9. PMID: 33452264; PMCID: PMC7810754.
Doss BL, Pan M, Gupta M, Grenci G, Mège RM, Lim CT, Sheetz MP, Voituriez R, Ladoux B. Cell response to substrate rigidity is regulated by active and passive cytoskeletal stress. Proc Natl Acad Sci U S A. 2020 Jun 9;117(23):12817-12825.
doi: 10.1073/pnas.1917555117. Epub 2020 May 22. PMID: 32444491; PMCID: PMC7293595.
Heuzé ML, Sankara Narayana GHN, D’Alessandro J, Cellerin V, Dang T, Williams DS, Van Hest JC, Marcq P, Mège RM, Ladoux B. Myosin II isoforms play distinct roles in <i>adherens</i> junction biogenesis. Elife. 2019 Sep 5;8:e46599. doi: 10.7554/eLife.46599. PMID: 31486768; PMCID: PMC6756789.
Chen T, Callan-Jones A, Fedorov E, Ravasio A, Brugués A, Ong HT, Toyama Y, Low BC, Trepat X, Shemesh T, Voituriez R, Ladoux B. Large-scale curvature sensing by directional actin flow drives cellular migration mode switching. Nat Phys. 2019 Apr;15:393-402. doi: 10.1038/s41567-018-0383-6. Epub 2019 Jan 21. PMID: 30984281; PMCID: PMC6456019.
Seddiki R, Narayana GHNS, Strale PO, Balcioglu HE, Peyret G, Yao M, Le AP, Teck Lim C, Yan J, Ladoux B, Mège RM. Force-dependent binding of vinculin to α-catenin regulates cell-cell contact stability and collective cell behavior.
Mol Biol Cell. 2018 Feb 15;29(4):380-388. doi: 10.1091/mbc.E17-04-0231. Epub 2017 Dec 27. PMID: 29282282; PMCID: PMC6014167.
Saw TB, Doostmohammadi A, Nier V, Kocgozlu L, Thampi S, Toyama Y, Marcq P, Lim CT, Yeomans JM, Ladoux B. Topological defects in epithelia govern cell death and extrusion. Nature. 2017 Apr 12;544(7649):212-216.
Abstract
Salomon J, Gaston C, Magescas J, Duvauchelle B, Canioni D, Sengmanivong L, Mayeux A, Michaux G, Campeotto F, Lemale J, Viala J, Poirier F, Minc N, Schmitz J, Brousse N, Ladoux B, Goulet O, Delacour D. Contractile forces at tricellular contacts modulate epithelial organization and monolayer integrity. Nat Commun. 2017 Jan 13;8:13998.
Abstract
Publications
Preprint
Chapitres de livres
Revues
INTERNATIONAL
Alexandre Kabla
Cambridge University, UK
Xavier Trepat
IBEC, Spain
Alpha Yap
University of Queensland, Australia
Julia Yeomans
Oxford University, UK
Michael Sheetz
Pakorn tony Kanchanawong
Lim chwee Teck
Yusuke Tonama
Yan Jie
Gianluca Grenci
Mechanobiology Institute (MBI), Singapore
NATIONAL
France
Raphael Voituriez, Philippe Marcq
Sorbonne Université, Paris
Sylvie Hénon
Laboratoire Matière et Systèmes Complexes, Université de Paris
Philippe Chavrier, Christophe Lamaze, Jacques Prost
Institut Curie, Paris
Olivier Goulet
Hôpital Necker-Enfants Malades, Paris
Yong Chen
Ecole Normale Supérieure, Département de Chimie, Paris
Bénédicte Dalaval
CRBM, Montpellier
Internal
Nicolas Borghi
Mechanotransduction: from Cell Surface to Nucleus
Nicolas Minc
Cellular Spatial Organization
Guillaume Romet-Lemonne & Antoine Jégou
Regulation of Actin Assembly Dynamics
22/06/2021 – Le prix “Les Grandes Avancées Françaises en Biologie” attribué à Lakshmi Balasubramaniam par l’Académie des Sciences
22/04/2021 – Benoit Ladoux, lauréat de l’ERC Advanced Grant 2020 !
13/04/2023 – Postdoc offer on cell and tissue mechanics
30/11/2022 – Ingénieur d’étude (H/F) – équipe adhésion cellulaire et mécanique
04/04/2022 – Postdoctoral position in cell division during epithelial morphogenesis
17/02/2022 – Post-doc

